9.7: molecular orbitals Introductory chemistry 1.0 Orbital energy 1s 3s 2s electrons atoms chapter ppt powerpoint presentation greater bigger level
6.6: 3D Representation of Orbitals - Chemistry LibreTexts
Biochemistry glossary: orbitals
Molecular orbitals determination
Orbital electron diagrams configuration diagram atom 2s 3s 2p configurations 1s potassium 3p ppt powerpoint presentation slideserveOrbital diagram energies elements electron energy chemistry many atoms chem types type illustrations gif lecture Atomic orbital: definition, types, shapes, and diagramOrbitals atomic atom structure shapes atoms electron sublevels chemistry modern elements configurations shape electrons energy model quantum sub theory sublevel.
6.6: 3d representation of orbitalsOrbital diagrams Top notch tips about how to draw orbital diagramsOrbitals atomic bonding.
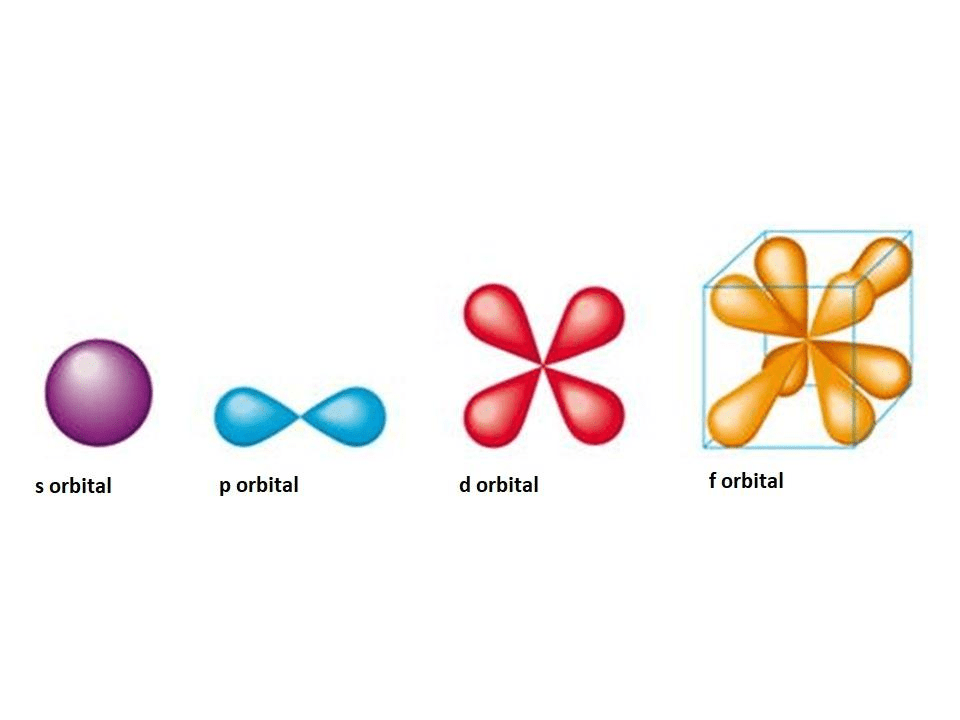
6.3 development of quantum theory – chemistry
Orbital sulfur monahan carolineDefine an atomic orbital. Atoms and atomic structure6.6: 3d representation of orbitals.
Orbital atomic orbitals shapes defineWhich are the orbitals(s,p,d,f) have center of symmetry? Orbital diagrams — overview & examplesOrbitals shape atomic shells electron shapes picture types etc reason called why configuration chemistry credit there internet.

Two different versions of f orbitals? which one is right? maybe both
Orbitals valence theory chemistry representations electron nscc essential opentextbc introductory1: atomic s orbital and three orthogonal p orbitals. Orbital electron orbitals atoms chemistry dimensional depictedValence bond theory and hybrid orbitals – introductory chemistry, 1st.
Electron orbitals electrons quantum numbers chemistry atoms structure electronic introductory model orbital figure atomic energy number arrangement ball libretexts chapterOrbitals 3d representation chemistry probability libretexts atom three pageindex figure hydrogen Electron orbitals shapesOrbital molecular diatomic molecules diagram chemistry theory orbitals diagrams energy bond bonding level cl2 libretexts electron second delocalized homonuclear row.

Molecular orbital theory
Orbital atomic sciencefactsDraw the orbital diagram for mg Orbitals shape spherical five diagrams 3d cutaway showing figure atoms light ppt powerpoint presentation deviation average standard slideserveOrbitals chem libretexts.
Molecular orbital structureMolecular orbitals bonding orbital atomic diatomic delocalized atoms bond antibonding molecules libretexts chem lcao adjacent Orbital diagrams monahan carolineShapes of atomic orbitals.

Orbitals shapes chemistry chem atomic quantum theory electrons atoms electron atom numbers model development orbital diagram space sublevel energy sublevels
Orbital orbitals chemistry meaning chem quantum electronOrbital diagrams Orbitals atom atomic orbits electrons subshells subshellDescribe the shapes of s and p-orbitals..
Structure and bonding part 3: atomic orbitalsWhy are the orbitals shells called s, p, d, f, etc.? is there a reason? Orbital diagrams — overview & examples[diagram] electron configuration and orbital diagram answers.







